MS Ultrasonic: Ultrasonic technology as the optimal welding solution in medical technology
This enables optimal welding, punching, sealing, cut-off welding and riveting of thermoplastics, textiles and nonwovens as well as cutting of food and other products.
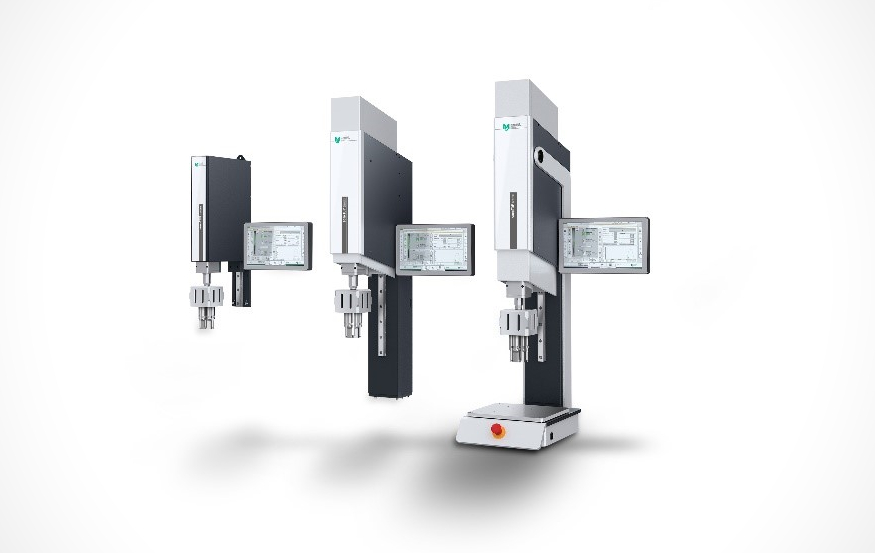
The company's more than 30 years of experience in the development and manufacture of high-quality and innovative ultrasonic components, coupled with expertise in drive and control technology, is also being applied to the medical technology sector. This means that customers receive ready-to-integrate systems with maximum precision from a single source or ready-to-use series machines (see picture).
 |
|
Due to the high requirements in the medical sector, ultrasonic welding is a very good choice for processing. A high degree of tightness, strength, optimum wearing comfort and minimal particle formation in the welding process are guaranteed. Due to our complete process monitoring and full documentation, this is the optimal solution for processing plastic parts in medical technology with any high quality requirement for a long service life.
In addition, MS Ultrasonic Technology Group is certified according to ISO 9001, ISO 13485 and ISO 14001 and is therefore familiar with high quality standards as well as quality assurance with competent project management, especially for medical technology.
Medical devices that are used in a sterile environment must have a hygiene status that corresponds to their intended use. The prerequisite to ensure this permanently is a suitable processing method (FDA, ISO 14644-1 and -2, MDR Directive, EMA) to which ultrasonic technology belongs. The MS sonxTOP series machines are also suitable for use in clean rooms, as they do not require compressed air.
-

MS Ultrasonic Technology Group
Ultrasonic welding of plastics, single-purpose equipment and tools, ultrasonic equipment, plastic welders.
Expansion of Manufacturing Capabilities at the ENGEL Kaplice Plant
9.5.2025 The ENGEL plant in Kaplice has expanded its manufacturing capabilities to include final assembly of machines. This step marks an important milestone in the development of the global production network and increases flexibility in responding to...














-jpg-280x250.jpg)


















