Fluid Dispensing for Medical Device Manufacturing with Nordson EFD
The medical device and life sciences industry must meet stringent regulations for quality and product consistency, making process control a critical issue. All materials and manufacturing processes – including machining, assembly, and packaging – must be documented for complete traceability and process validation.
This is critically important with fluid dispensing applications in the assembly of medical devices, point-of-care testing (POCT) and near-patient testing (NPT) products, and other life sciences applications such as medical wearables, which require accurate and consistent deposition of fluid amounts of UV-cure adhesives, cyanoacrylates, silicones, and other fluids in their manufacture.
Regardless of the application, the fluids being dispensed and the dispensing technique, in addition to maintaining the quality standards required, the dispensing method must also meet requirements for volume throughput and cost efficiency. Any increase in production volume requirements when relating to assembly, is often the key driver that necessitates the move to a more efficient dispensing system.
 | |
| Fig.1: Optimum Class VI dispensing components include 3cc, 5cc, 10cc, and 55cc barrels, pistons, end caps and tip caps. |
Responding to COVID-19
This is particularly true now as medical product manufacturers worldwide have, over the past three years, increased production of critical supplies and medical devices to address COVID-19 (SARS-CoV-2). Dedicated to helping manufacturers increase production capacity to meet this growing demand, fluid dispensing equipment manufacturers have come forward with precise and safe dispensing solutions to mass produce ventilators, diagnostic test kits and other medical devices.
Responding to requests early in the COVID-19 pandemic, Nordson EFD supplied multiple automated, robotic fluid dispensing systems to mass produce a small sub-assembly inside Ventec Life Systems’ ventilators. Ventec’s critical care ventilators are portable and have provided front-line medical professionals with the systems they need to fight COVID-19. The sub-assembly application required bonding two components together using a UV-cure acrylic.
Prior to the pandemic, Ventec was using manual dispensing techniques. COVID-19 forced the company to quickly expand to meet a 180% increase in production volume per month. This was achieved, in part, by using the automated robotic fluid dispensing systems provided by Nordson EFD.
 | |
| Fig.2: Bar code scanning of medical device components permits access to stored dispensing programs, reducing human error. |
Another high-priority COVID-19-related application provided by Nordson EFD involved jetting medical reagents onto diagnostic test strips and bonding the housing of test cards for COVID-19 test kits. Nordson EFD provided proprietary PICO Pµlse® jetting systems for these applications, due to its fast-dispensing speed and extremely high precision.
These applications are characteristic of manufacturers who needed to scale the sophistication of their fluid-dispensing processes to meet COVID-19 increased-production requirements. In most high-volume manufacturing environments, automated and semi-automated fluid dispensing applications may be in use, dependent on the throughput volume and quality standards required at any stage in the assembly process.
 | |
| Fig.3: xQR4IV needle valve dispensing UV-cure material onto a medical device. |
Scaling Dispensing Automation to Meet Production Requirements
Many medical device manufacturers started out with manual squeeze bottles and medical syringe dispensing. Then, as production volumes increase, some progress into employing more controlled approaches with precision benchtop fluid dispensers, pneumatic valve systems or in-line robotic dispensing systems, for at least part of their fluid dispensing.
There are a number of factors that would support adopting a more efficient and controlled dispensing method as a better business solution:
a) Shot-to-shot repeatability and accuracy are improved as a more automated and controlled dispensing approach is employed.
b) Increased productivity is clearly a benefit that comes with increased automation. For example, the same worker who manually assembles 800 parts during an eight-hour shift can assemble 1,000 to 1,200 parts with the assistance of a pneumatic fluid dispenser.
c) Part quality improves when switching from manual squeeze bottle dispensing to air-powered dispensing, and further along to in-line automated dispensing, because operator-to-operator variance is significantly reduced. The ability to set the time, pressure, and other dispensing parameters for an application improves process control and ensures the right amount of fluid is placed on each part.
d) Rework and reject rates lessen when upgrading to more automated dispensing solutions thus improving the yield of the manufacturing lines and greater profitability to the manufacturer.
e) The amount of assembly fluid used decreases significantly when using a more controlled method of dispensing. Switching from a rudimentary manual dispensing process, for example, to a pneumatic dispenser can cut the amount of fluid used typically from 50 to 70 percent due to the improved accuracy of the deposit.
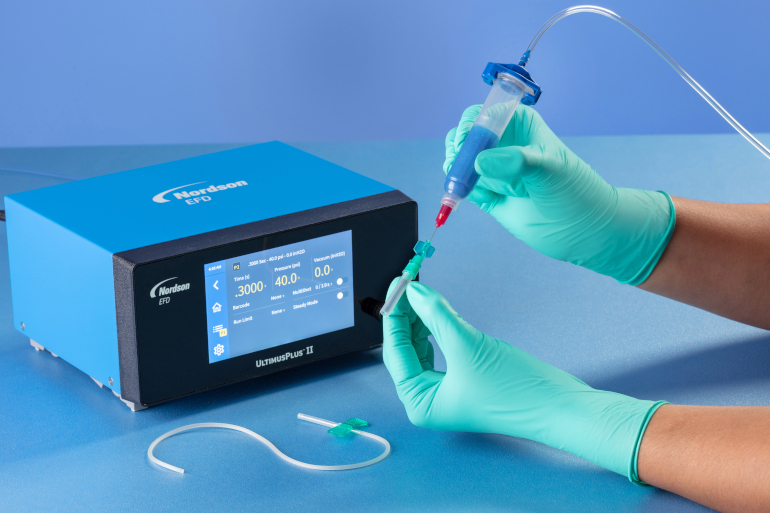 | |
| Fig.4: Manual application of assembly fluid onto a catheter using an UltimusPlus fluid dispenser and Class VI disposable syringe. |
Medical device manufacturers can benefit by taking a closer look at their production requirements and embracing a more controlled and automated fluid dispensing capability. It is critical, however, to consider each of the five points above, as they represent the actual cost-to-benefit factors influencing fluid dispensing processes.
- autor:
- Nordson EFD




















